Relative atomic mass of an element is the average value of all the known isotope atomic weight relative to the proportional abundances.
Relative atomic mass is more useful in chemistry than its simple atomic mass of element because simple atomic weight does not consider existence of
isotopic forms of elements. The relative abundance of isotopic element is applied to determine relative atomic mass. Example isotopic element X contained Y% of zªX and
b
W% of zX, the relative atomic mass of element X is determined as given below.
RAM of X=[Y/100 × a]+[w/100 × b]
NB:Relative abundances (Percentages i.e. Y% and W in this case, are multiplied by their respective mass numbers
Since y + w = 100
The length of peaks of each isotopic atom is applied to find the relative atomic mass. Each atom have a peak due to different mass charge ratio m/e.
Problem #01
 (chlorine-37). The abundance of chlorine-35 is found to be 75% while that of chlorine-37 being 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
(chlorine-37). The abundance of chlorine-35 is found to be 75% while that of chlorine-37 being 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
Solution
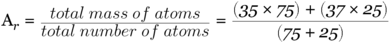

 (to 1 decimal place)
(to 1 decimal place)
RAM=(69×63) + (31×65)
(69+31)
.:RAM = 63.6
The RAM of copper is 63.6.
Relative atomic mass is more useful in chemistry than its simple atomic mass of element because simple atomic weight does not consider existence of
isotopic forms of elements. The relative abundance of isotopic element is applied to determine relative atomic mass. Example isotopic element X contained Y% of zªX and
b
W% of zX, the relative atomic mass of element X is determined as given below.
RAM of X=[Y/100 × a]+[w/100 × b]
NB:Relative abundances (Percentages i.e. Y% and W in this case, are multiplied by their respective mass numbers
Since y + w = 100
The length of peaks of each isotopic atom is applied to find the relative atomic mass. Each atom have a peak due to different mass charge ratio m/e.
RAM of X=(Y×a)+(Z×b)+(W×c)+(q×d)
(Y+Z+W+q)
Problem #01
 (chlorine-37). The abundance of chlorine-35 is found to be 75% while that of chlorine-37 being 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
(chlorine-37). The abundance of chlorine-35 is found to be 75% while that of chlorine-37 being 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
In calculating the relative atomic mass, Ar, of chlorine:
Note: that the answer appears to be more closer to 35 than it is to 37. Since the chlorine-35 isotope is much more abundant than the chlorine-37 isotope.
Problem #02
Copper has relative abundances of 69% and 31% for 63 and 65 mass numbers respectively.
Calculate the Relative Atomic Mass of copper, keep your answer to one decimal place.
Solution;
RAM of Copper= Summation of the products of Relative abundances with their respective Atomic masses of copper divide by Summation of their Relative abundances.
(69+31)
.:RAM = 63.6
The RAM of copper is 63.6.


No comments:
Post a Comment
Feel free to share your views